Seeing an Electron
(Text by Milo Wolff. Animation by Winston Wolff) I want to show you a very interesting web animation of an electron. But first let me explain something about the
structure of the electron. Then when you are finished reading, observe the animation below.What is an electron? Never in history has anyone been able to even imagine the structure of an electron. Until
very recently, the electron was thought to be a 'point' made of an unknown, undefineable substance called 'charge'. Since a point has no dimensions, there is no structure and nothing to see.
The electron is a wave structure. Recently this structure was found (see: A Wave Structure for the Electron) to be two concentric spherical moving quantum waves. One wave moves inward and one wave moves outward. This spherical wave pair has the familiar properties of an electron which we call mass and charge, as
well as all other properties. When you look below, for this first time in history you can see the radial amplitude of these electron waves moving on your screen. You will see three diagrams.
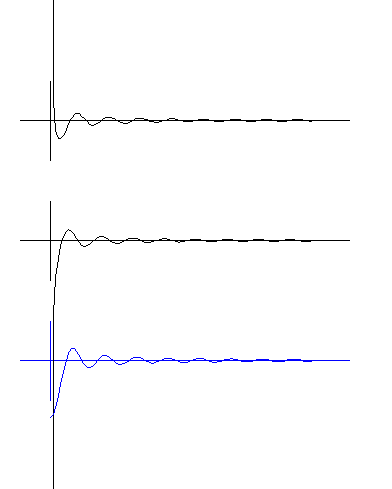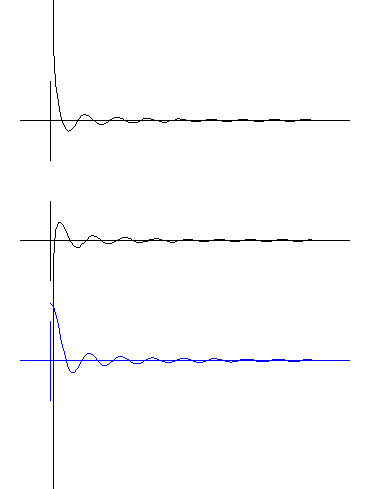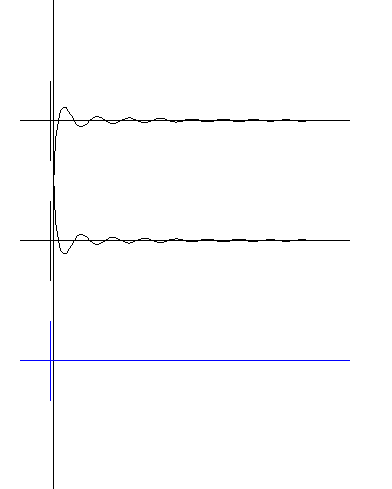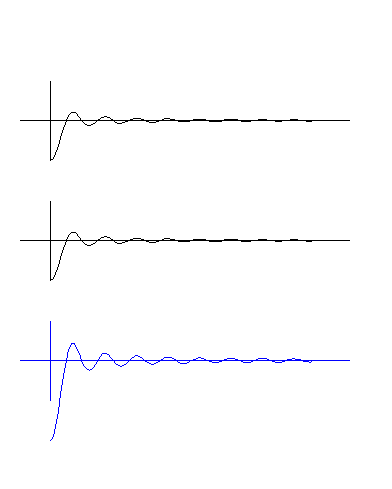
These diagrams are mathematical plots of the IN and OUT waves and the SUM
of both waves. The plots are the amplitudes of the waves drawn along a radius out from the electron center (at the left side). (Be patient - the mathematical program doesn't start instantly). You will see that the farther from the center, the smaller the amplitudes become. This is like familiar sound or light waves or even water waves which become weaker as they move outward. Quantum waves are very tiny. In this diagram they are amplified a hundred, million, million times (10
14). You have to imagine them smaller - but that is not difficult.
|
In the TOP DIAGRAM, observe the wave moving OUTward. On the left, where the radius is zero, the amplitude becomes + infinite (runs to the top of the
screen).In the MIDDLE DIAGRAM, observe the wave moving INward. Where the radius is zero the amplitude is - infinite (runs to the bottom of the screen). The blue BOTTOM DIAGRAM is
the SUM of the two waves. That is, the two amplitudes are added together, as always happens when two waves are at the same place. The sum of the waves is the radial amplitude of
the real electron. Watch carefully how the sum wave moves. The wave does not move inward or outward, it goes up and down. That is, it becomes a standing wave. Standing waves
also occur for light and sound waves; for example on a violin string. The waves on the string can go up and down but not run along the string because of the posts. The electron, and
other charged particles, are structured of standing spherical quantum waves |
|
|
Notice the behavior of the sum wave when the radius is near zero. The amplitude of the wave is not
infinite at zero radius. Instead you notice it reaches a size of about + or - one inch on the screen. This is an important difference between the wave structure theory compared to the old point theory of the
electron. In the point theory, electric amplitudes became infinite at zero radius. This is wrong because laboratory measurements show finite amplitudes. The wave structure, as shown here, gives the right result.
Where are the orbits? For many (too many) years people imagined atoms as point electrons orbiting around a nucleus. This myth, obviously imitating our planetary system, was shown wrong by quantum
theory more than sixty years ago. For example in the hydrogen atom, quantum theory predicts the electron presence as a symmetrical spherical cloud around the proton. Some physicists concluded that
the point bits of matter were still there, even though quantum theory contains no notion of point particles. The old myth dies hard!
Actually, in the H atom both the electron wave-structure and the proton have the same center.
The electron's structure can be imagined like an onion - spherical layers of waves around a center. The amplitude of the waves decreases like the blue standing wave in the bottom diagram. There are
no point masses - no orbits, just waves. Animated Particles. This diagram is the first animation of a basic particle for viewers on the web. Is
this Important? Or just a neat gimmick to make fuzzy physics come to life? Yes! It illustrates a newly recognized essential
property of a particle - communication. In order for particles to interact with each other, it is essential they communicate their position, direction, and presence to each other. The
dynamic wave-structured electron can do this. The old static point particle cannot. It is wrong. Understanding properties of the particles by animation will have a high priority in the future.
This animation was produced by my son Winston. You can see that his work making Star Wars video games like "Dark Forces" of recent best-seller fame, has scientific application. Thus I am doubly
proud that he was able to program it and put it on this web page. It required a lot of planning and skill. I am very grateful for his innovative help in making this science page for you. Return to the index page.
|
